Medical Devices Webinar by Asphalion

Vigilance MD PSURs for Medical DeviceПодробнее

Regulatory tips for medical device and IVD developersПодробнее

Clinical Evaluation of Medical Devices according to EU MDR 2017 745Подробнее

MDR postponed an opportunity that cannot be missedПодробнее

Medical Devices Webinar - Stability - 01/06/2023 - ENПодробнее

Webinar Farmaforum - Reglamento EU/2017/745 de productos sanitarios: la nueva realidadПодробнее

FDA Regulatory Affairs Webinar - AsphalionПодробнее

SYS-027 Purchasing Procedure WebinarПодробнее

Webinar : How to Meet FDA Cybersecurity Standards for Medical Devices | QualysecПодробнее

Regulatory Procedures for AI in Medical Devices - Webinar by RSIP Vision September 16Подробнее

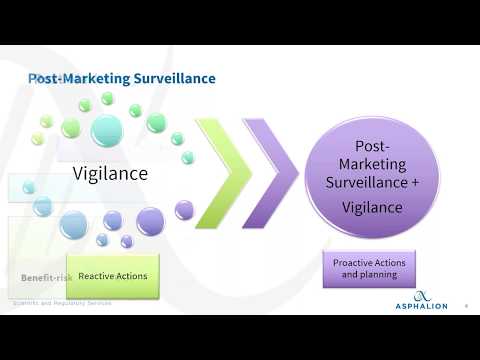
Post-Marketing SurveillanceПодробнее
JoinHealth | Spring welcome webinars 2024 | "Understanding Medical Devices Regulation"Подробнее

Medical Devices Webinar - Regulation, Advances and Perspectives - 23-11Подробнее

Webinar: Medical Literature MonitoringПодробнее

EU Exit and post-transition guidance, Regulation of Medical Devices Webinar - October 2020Подробнее

Webinar: Evidence-Based Approaches for the Biological Safety Assessment of Medical DevicesПодробнее

Webinar: Introduction to US FDA Medical Device Regulations (510k, De Novo, IDE, CAPA, eMDR)Подробнее

Medical Devices Regulations Webinar - 24 January 2023Подробнее
