ICH GCP E6 R2 Gap Analysis | Andy Lawton

Quality Tolerance Limits (QTLs) | ICH E6 R2 | Andy LawtonПодробнее

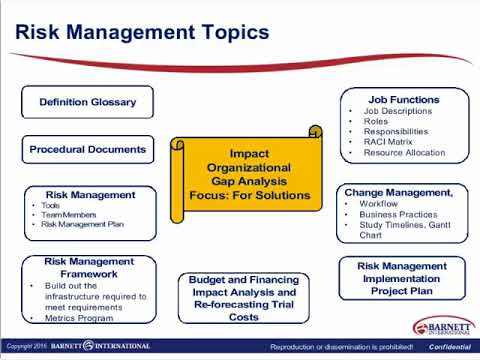
Final ICH GCP E6 R2: Implementing Risk Management Approaches for Compliance TrailerПодробнее
Вебинар: "Изменения в требованиях ICH GCP E6 (R2)"Подробнее

4 Key Components to Achieving Compliance with ICH E6 R2 FINAL1Подробнее

Changes in ICH GCP with the Upcoming Revision 3Подробнее

Final ICH GCP E6 R2: Implementing Risk Management Approaches for Compliance TrailerПодробнее

Change is Opportunity! ICH GCP E6 (R2) — 1 Mar 2018Подробнее

Final ICH GCP E6 R2: Impact on Clinical Data Management TrailerПодробнее

ICH-GCP & Principles of ICH GCP- E6 and E6(R2) Poster Presentation By Steeve Branden WoodПодробнее

AQC Webinar - Inspection Preparation With a Risk-Based Approach Consistent with ICH E6 (R2) Requ...Подробнее

Final ICH GCP E6 R2 Addendum TrailerПодробнее

Final ICH GCP E6 R2 Addendum TrailerПодробнее

RBM Webinar 2 - What Could Go Wrong? ICH E6 R2, Investigative Sites and Risk AssessmentПодробнее

Final ICH GCP E6 R2 Addendum: Overview of Changes Impacting Sponsors/CROs/Clinical Investigator/SiteПодробнее
