EMA Perspectives/Clinical Trials and Frailty

The EMA Perspective: Rapid Clinical Research Approval & Interpretation Data During COVID-19 PandemicПодробнее

EMA workshop on patient experience data in medicines development and regulatory decision-makingПодробнее

2021 T1D Workshop- EMA Perspective: Clinical endpoints and validated surrogatesПодробнее

PANEL: Frailty as an Outcome in Clinical trialsПодробнее

Clinical Trials in the EU - short versionПодробнее

The EMA perspective on drug approval process in the era of precision medicineПодробнее

Clinical trials in times of Corona - FDA and EMA UpdateПодробнее

Overview of the European Medicines Agency (EMA), Part 1 of 3Подробнее
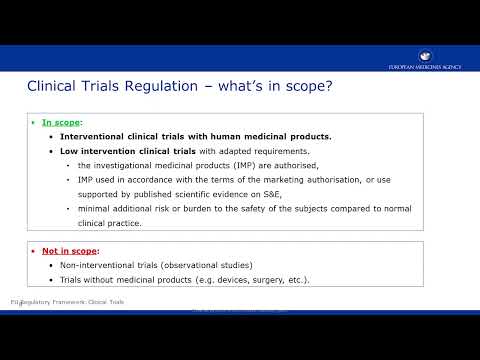
ICTD 2021 - EMA Clinical Trial Information System (CTIS) for platform trials - Noémie ManentПодробнее

Frailty Seminar Series: Frailty as an Outcome in Clinical Trials (Panel)Подробнее

2021 T1D Workshop- EMA perspective | Peter MolПодробнее

Are frailty scores being used in clinical trials? How?Подробнее

Webinar on transparency rules for the EU Clinical Trials Information System CTISПодробнее

Integrating Frailty Into Clinical PracticeПодробнее

Accelerating clinical trials in the EU (ACT-EU) by I. Den Rooijen (EMA)Подробнее

HAI Europe - Dr. Hans-Georg Eichler (EMA - European Medicines Agency)Подробнее

Clinical Trials Information System (CTIS): Walk-in clinicПодробнее

Longeveron's Dr Joshua Hare talks frailty, Alzheimer's and cell therapy clinical trials.Подробнее
