Advancing Transparency and Regulatory Science Activities on Risk Evaluation and Mitigation Strategy

Risk Evaluation and Mitigation Strategies (REMS) Compliance ProgramПодробнее

Science and Decisions: Advancing Risk AssessmentПодробнее

Regulatory Education for Industry (REdI) Annual Conference 2022 - Day 2 - Part 3Подробнее

FDA's Sentinel Initiative - Pharmacovigilance 2020Подробнее

Regulatory Science StrategyПодробнее

ICH Q12 Guidance and Emerging Technology Program (11of16) Generic Drugs Forum 2020Подробнее

About FDA’s Regulatory Science ProgramПодробнее

Advancing Generic Drug Development: Translating Science to Approval - Day 1 – Keynote and Session 1Подробнее

Driving Biomedical Innovation by Advancing Regulatory Science at FDAПодробнее
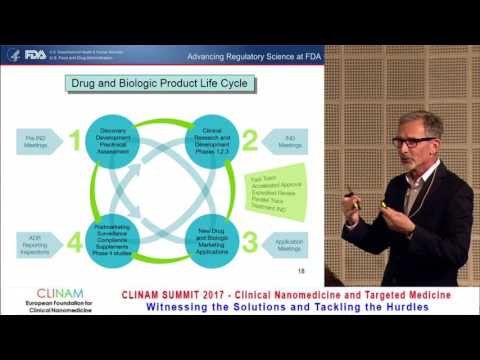
What is Regulatory Science?Подробнее

MDUFA IV: Accessing and Using Real-World and Postmarket Data for Regulatory Decision-MakingПодробнее

Division of Applied Regulatory Science in FDA’s Center for Drug Evaluation and ResearchПодробнее

Advancing risk assessment science: Environment (19 Sept, part 2)Подробнее

What Science Got to Do at the Regulatory AgencyПодробнее

Pharmaceutical Quality Symposium 2023: Quality, Supply Chain & Advanced Manufacturing – D1 – Part 4Подробнее

FDA CDER Regulatory Science: The Importance of Partnership and ConsortiaПодробнее

AI, Open Science, and Transparency in Regulatory ScienceПодробнее

How is Risk Mitigation and Risk Assessment Happening?Подробнее
